
The conceptual structure of the SFB 1280

The SFB 1280 brings subprojekts together, that are superficially surprisingly different. Its similarities have been incorporated into research questions: seven comprehensive hypotheses that will be investigated by all associated projects from different perspectives. The hypotheses set a conceptual framework and function as experimental aims for the projects. The set of fundamental questions are complemented by seven hypotheses from the two Focus Groups, specialized on the fields learning dynamics and neuroimaging.
Both learned inhibition and forgetting characterize extinction learning as parallel events.
The nature of extinction learning
As outlined above, the results of behavioral studies on extinction learning are usually interpreted as either reflecting an unlearning (or forgetting) of the CS-US association or an acquisition of inhibition that stops the previously acquired behavior. The figure depicts the two theoretical positions as positive or negative exponential curves of associative strengths across trial number. These curves are accompanied by schematic depictions of spike frequency changes of BLA neurons of the rodent amygdala during initial acquisition and extinction learning. To compile this visualized overview are idealized curves of changes of single unit responses during acquisition and extinction learning of four publications have been transformed. It is visible that the electrophysiological data from the BLA perfectly match the predictions of learning theory. The insight from electrophysiology has implications for learning theory: It could imply that extinction learning is a parallel unfolding of both learned inhibition and forgetting. If this is true, we expect signatures of both processes also in other neural structures, species, and types of extinction learning (A01, A02, A04, A06, A08, A09, A14, A15). Inhibition and forgetting as parallel events should then also be visible in behavioral data. Also the Focus Group “Learning Dynamics” F01 will harvest SFB 1280 data on this issue.
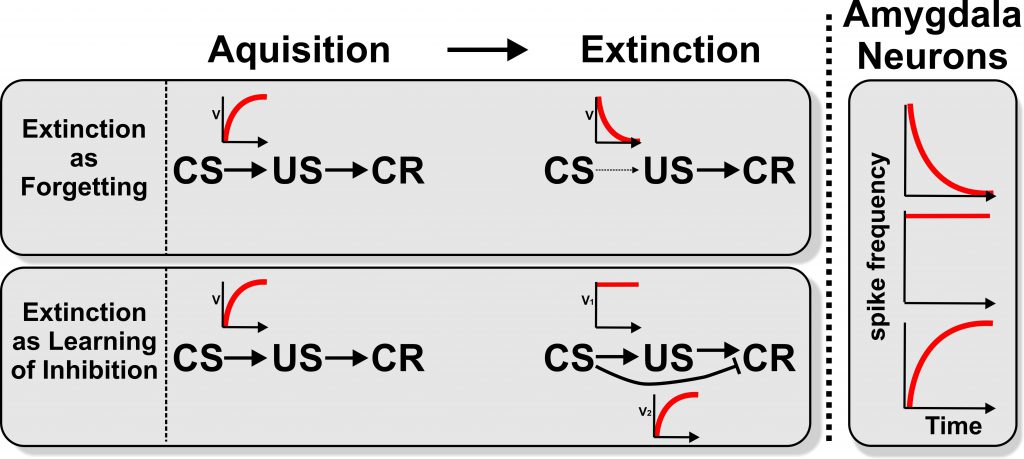
Schematic presentation of learning processes and neural amygdala signals during first learning and extinction. During acquisition an association between a conditioned stimulus (CS) and an unconditioned stimulus (US) is learned. Thereby the CS gains a predictive power which leads to a conditioned response (CR). The red curves indicate a schematic development of the strength of association (V) between the CS and US and CS and CR over time.
Appetitive and aversive extinction procedures differ less at the behavioral level but still can vary substantially at the neural level. The specific biological salience of CS and US affect excitatory and inhibitory learning, including extinction efficacy.
The vast majority of studies on the neural basis of extinction learning use fear extinction paradigms – particularly in the form of Pavlovian aversive conditioning paradigms. Previous studies compared extinction learning in appetitive and aversive experimental designs to examine if results from fear conditioning can be generalized to appetitive conditioning. The results suggest that appetitive and aversive behavioral studies of extinction reveal mostly overlapping results. Similarly, some neurobiological studies in humans that compare PFC activation during appetitive and aversive operant conditioning and subsequent extinction revealed an overlapping pattern: In both cases successful extinction was associated with an increased activation of frontopolar orbitofrontal cortex in conjunction with a decreased activity of the amygdala and the right anterior cingulate cortex. However, it is unknown if these similarities also hold in different healthy populations and patients’ groups. This is why several human brain imaging projects within this SFB will compare behavioral and neural mechanisms underlying aversive and appetitive learning in different populations and learning paradigms (A03, A09, A11, A16).
Such knowledge is urgently needed, because when going into details, important differences do indeed become visible. One key difference is the endogenous cannabinoid system that differently modulates aversive and appetitive extinction procedures and is, among others, found in cortex, hippocampus, and amygdala. A further difference between extinction of aversive and appetitive conditioning is due to different coding properties of dopaminergic transmission. Studies revealed that dopamine neurons in the ventral tegmental area (VTA) showed phasic excitation after exposure to either reward predicting cues or to primary rewards. This is consistent with models of reward prediction coding – a property analyzed in project A15. GABAergic neurons of the VTA, however, were excited by aversive stimuli and could subsequently suppress dopaminergic cells. Thus, appetitive and aversive dopaminergic signaling is mediated by distinct neuronal VTA populations and so possibly by two diverse systems.
The amygdala also exhibits differential activation patterns during appetitive or aversive conditioning tasks. Studies found that amygdalar neurons in monkeys specialize for the appetitive or the aversive valence of stimuli change their firing properties in tight correlation with the behavior of the animal when stimulus valence is extinguished or reversed. Hippocampal neurons change their memory valence engram correlated with changing their cellular partners within the amygdala. Neurons of the BLA, however, stay mostly tuned to a fearful or rewarding association. These data provide a network account of the differential representation of appetitive and aversive condition events. To further elucidate this knowledge, we will analyze data from human aversive and appetitive learning paradigms in the Focus Group “Neuroimaging” (F02) with respect to structural and functional connectivity changes with the extinction network as predictors for extinction learning.
Finally, the SFB 1280 would like to emphasize a further facet of associative learning that is not captured by the dichotomy of aversive and appetitive conditioning: Preparedness is the catchword here and it concerns the evolutionary significance of CS and US which shapes excitatory and quite possibly also inhibitory learning processes. Hence, the salience of stimuli affects learning in terms of speed and strength of the CS-US association, the magnitude of conditioned responses, and possibly resistance to extinction. Consequently, several groups of the SFB 1280 will address this by implementing different types of biologically salient CS or US in some of their experiments (A10, A11, A12, A13, A18).
The distinction between cues and context is learned. This learning requires the hippocampus due to its role in storing memories of past experiences. Prefrontal mechanisms interact with the hippocampus to translate these context-dependent experiences into behavior.
How does context affect extinction learning?
There is overwhelming evidence that extinction learning is more context-dependent than the original acquisition. Without context-dependence there would be no renewal effect after extinction learning. The SFB 1280 aims to study context-dependence and advance the understanding thereof. In this regard, it will be guided by three questions:
What is context?
One possible assumption is that stimuli have certain properties that allow for their a priori classification into discrete cues on the one hand and contextual stimuli on the other. For instance, cues are typically discrete and can change rapidly, whereas context is thought to be diffuse and slowly-changing. However, “contexts are ill-defined stimuli” (Holland and Bouton 1999, S.195) and an a priori distinction between contexts and cues cannot account for several important experimental findings.
An alternative view is that there is no categorical difference between discrete cues and contextual information during associative learning, i.e., all sensory inputs drive learning to some extent. Experimental evidence suggest that indeed the context is learned. The SFB 1280 hypothesizes that the distinction between cues and context is learned, i.e., the differences in contingency and contiguity as implemented by the experimenter lead to different responses when the stimuli are presented. The presentation of a cue elicits the conditioned response, whereas exposure to a context does not necessarily. The context does however have an modulatory influence on behavior.
What are the learning mechanisms underlying context-dependence?
A challenge for the learning hypothesis is that associative learning models in their simplest form cannot account for renewal. However, learning in these models is typically driven by a small number of inputs that are available at a given time-point. While this information is sufficient to model the learning and unlearning of the strong association between the CS and US, it is insufficient to model the more subtle and complex context-dependence of extinction learning. Project A14 will use computational modeling to test the hypothesis that associations are learned between any stimulus and the US, but only the associations with CS are strong enough to elicit the conditioned response. The associations with context are too weak to drive behavioral output directly, but sufficient to modulate it.
What is the neural basis of context-dependence?
Contextual information is thought to be provided by the hippocampus. Experimental evidence shows that context-coding in extinction requires the hippocampus, whereas classical conditioning does not necessarily. However, it remains unclear what specific role the hippocampus plays. Cheng (2013) suggested that the hippocampal involvement in a number of conditions is better accounted for by its function in episodic-like memory. Here, the SFB 1280 hypothesizes that the role of the hippocampus in episodic-like memory accounts for its role in context-dependence of extinction learning. The research group further assumes that the interaction between the hippocampus and the prefrontal cortex is necessary to translate past experiences into altered behavior during extinction (A01, A03, A04, A06, A08, A09, A10, A16).
Sensory cortical fields show similar extinction learning properties as the BLA. These cortical fields will also modulate their striatal territories similarly as the BLA modulates its corresponding CEA segments. Thus, during extinction activity patterns within both sensory cortical areas as well as corresponding dorsal striatal territories are altered.
The amygdala as a specialized corticostriatal system
The amygdala is a specialized limbic constituent that controls various instinctive, emotional and/or affective behaviors by its projections to subpallial systems. How can the neurobiological basis of amygdala-dependent processing of fear acquisition and extinction be translated to other forms of extinction learning that are cognitive and do not necessarily entail a limbic component? The BLA forms the ventromedial extension of the claustrum, which is the deepest part of the insular cortex. Based on embryology, neurochemistry, genetic analyses, morphology, connectivity, and topology, the BLA possibly corresponds to lamina V of the temporal and frontal cortex. Indeed, cortical lamina V neurons receive also sensory input from multimodal intralaminar nuclei as does the BLA. Similar to neurons of the BLA, sensory cortex lesions have no impact on fear acquisition but impair extinction learning and alter responses during fear acquisition and extinction. The CEA, on the other hand, is, by virtue of all available connectional, immunohistochemical, topological, and genetic evidences a specialized striatal region that selectively modulates autonomic motor outflow. Most CEA neurons are GABAergic and indistinguishable from medium spiny striatal cells. In addition, the striatum receives input from thalamic intralaminar nuclei that project also to amygdala and cortex. Thus, intralaminar nuclei provide multimodal information that calls for attention, orienting, and action selection to all three: amygdala, striatum, and cortex. Consequently, the dorsal striatum also plays a role in extinction learning that parallels that of the amygdala. This SFB therefore assumes that corticostriatal circuits show similar properties during extinction learning as the BLA-CEA-system. These corticostriatal loops correspond to parts of the uncharted territories of extinction learning with or without limbic contributions and will be studied in the SFB 1280 (A01, A02, A03, A06, A08, A09, A10, A11, A12, A16).
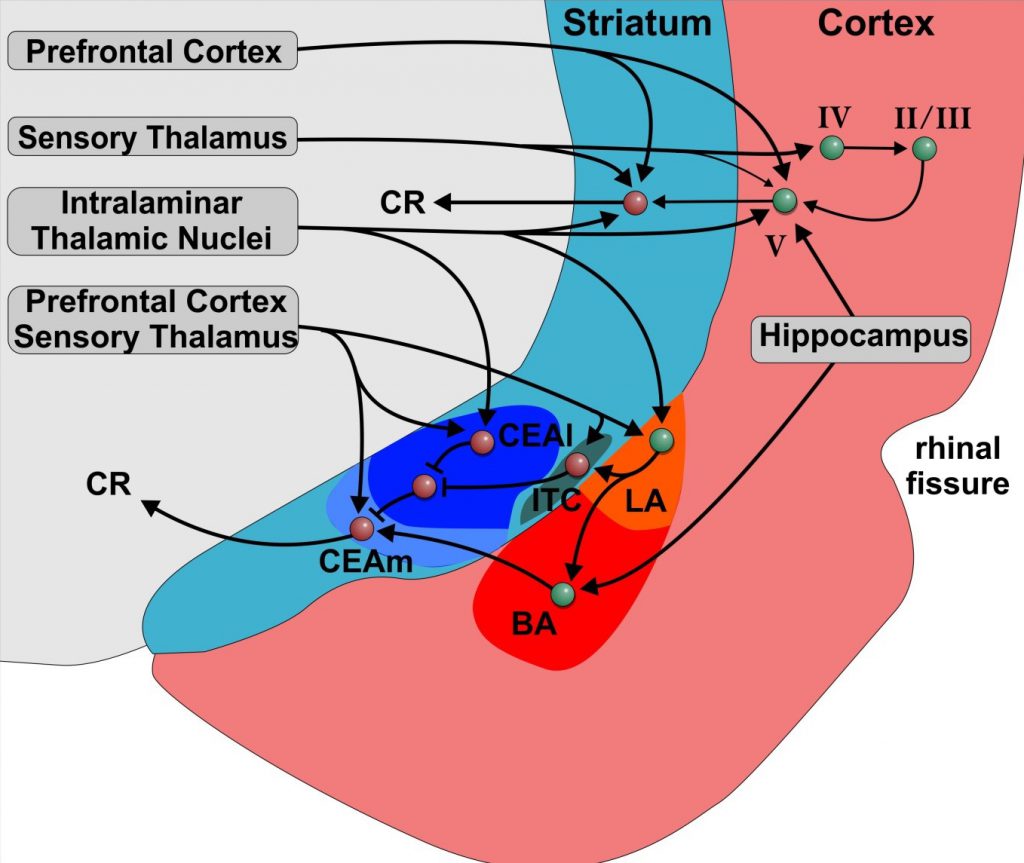
Schematic illustration of the amygdala and adjacent cortical and striatal regions in rodents. Warm colours indicate pallial/cortical regions and cold colours represent striatal areas. Roman numerals indicate cortex layers. The afferent connections of the cortex and the striatum as well as the interconnectivity show great parallels to the pallial and striatal amygdala. The striatal amygdala also partially includes pallial parts. Abbreviations: BLA: basolateral amygdala; CEA: central nucleus of the amygdala; ITC: intercalated cell cluster; LA: lateral nucleus of the amygdala; BA: basal amygdala; CEAl: lateral part of the central nucleus of the amygdala; CEAm: medial part of the central nucleus of the amygdala.
The cerebellum is part of the neural circuit underlying the different aspects of extinction, including contextrelated processes, conditioned fear, and all other forms of associative learning.
Cerebellum – terra incognita of extinction research
The cerebellum is possibly the largest neural terra incognita of extinction research. Cerebellar contributions to eyeblink conditioning extinction have been studied to some extent. But only recently novel reports on the role of the cerebellum in extinction of learned fear and in context-related processes of extinction started to be published. The same cerebellar circuit that is involved in acquisition of conditioned eyeblink responses is possibly also involved in extinction. The SFB 1280 hypothesize that the same principles apply for the acquisition and extinction of conditioned fear. Most importantly, it is likely that the cerebellum interacts with forebrain areas during extinction of conditioned eyeblinks. The cerebellum is anatomically and functionally connected with the PFC, the hippocampus, and the amygdala. Since the cerebellum and hippocampus interact during spatial navigation, a modulatory role of the cerebellum in context learning via hippocampal projections appears possible. Alternatively, the cerebellum may support attention shifts to context via its connections to dorsolateral PFC. The SFB 1280 hypothesizes that cerebellar areas involved in context processing are different from cerebellar areas related to extinction learning. PFC and/or hippocampus may also inhibit learned associations within the cerebellum involved in the expression of conditioned responses. All this has never been studied and several projects of the SFB are specifically focused on these kinds of questions (A05, A06). Finally, the cerebellum and the amygdala possibly directly interact during fear extinction. These and many further options now become conceivable with ongoing integration of the cerebellum into the known neural network of extinction learning. The SFB 1280 is a part of this endeavor with contributions from all human imaging projects (A02, A03, A05, A06, A08, A09, A10, A11, A12, A16) and the Focus Group “Neuroimaging” F02.
Neuroendocrine and immune activation differentially affect extinction consolidation and its retrieval. These effects are further modulated by the task-induced emotional arousal and context. The underlying mechanisms involve specific alterations in the extinction network and also apply to learned immune responses.
The neuro-endocrine-immune-interface in extinction-related processes
Extinction learning, its consolidation, and its retrieval are influenced by multiple neuromodulators. Within the SFB 1280 monoamines (e.g. dopamine, noradrenalin and serotonin), glucocorticoids, and immune messengers will be investigated. Dopamine is key for extinction since it is at the core of the prediction error signal. Previous work from members of the SFB 1280 could demonstrate that dopamine (DA) also influences appetitive extinction learning in spatial tasks in rodents and humans. The role of dopamine in extinction will therefore be investigated in projects A04 and A08. Serotonin modulates many neuronal functions such as cognitive appraisal and emotional responses and has been linked in particular to stress-induced affective disorders. Surprisingly, its role for fear learning and extinction has received little attention. Evidence for a role of serotonin for fear acquisition and extinction comes from human genetic and animal studies suggesting that serotonin levels are increased during fear conditioning, psychological stress and fear memory retrieveal. The role of serotonin in modulating neuronal circuits underlying extinction will be addressed in project A07 and A06. A07 will furthermore explore the interaction between stress and serotonin in modifying the signaling cascades within the amygdala during extinction learning. Noradrenaline (NA) from the locus coeruleus is mediating the impact of emotional arousal on neuronal excitability and is mostly known for its boosting effect on attention and memory consolidation. Fear-conditioning studies conducted in rodents have demonstrated a crucial role of noradrenaline (NA) in fear extinction. Experiments conducted by some of the SFB 1280 PIs revealed enhanced extinction when NA concentrations were pharmacologically boosted, and impaired extinction when NA-receptors were antagonized, the role of NA for extinction will be addressed in several projects (e.g. A04, A07, A08, A09) Extinction learning is also modulated by neuroendocrine and peripheral immune systems. Two lines of research specifically explore these systems in extinction-related processes in the SFB 1280. The first concerns stress as a potent modulator of extinction learning, consolidation, and retrieval. The stress-induced activation of the hypothalamus-pituitary-adrenal (HPA) axis and the sympathetic nervous system (SNS) influence learning and memory processes via modulation of the amygdala, the hippocampus, and the PFC. Stress exerts a phase-dependent effect on long-term memory with differential effects on consolidation and memory retrieval. Interestingly, the impact of stress on learning is modulated by the emotionality of the material to be learned and is furthermore influenced by the context in which learning takes place, indicating the need to test effects of stress in different types of learning paradigms. In a series of human studies, members of the FOR 1581 have tested the impact of acute stress on extinction retrieval using a renewal paradigm. These findings are in line with the hypothesis that the more emotional the memory trace is, the more stress affects this trace. These studies enable clinically-oriented research on stress effects on recovery phenomena such as reinstatement in healthy individuals and patients (see A09, A10, and A11). Fascinating, yet thus far completely untested implications for pain will also be analyzed. With respect to extinction consolidation, it was demonstrated that post-extinction stress leads to a more context-dependent extinction memory, which is associated with a more pronounced renewal effect. In contrast, pre-extinction stress made extinction less context-dependent which fits to clinical studies showing beneficial effects of cortisol administration on extinction-based treatments. The possibility of enhancing extinction success by administering a laboratory stressor will be tested in project A13. With regard to the immune system, pro-inflammatory cytokines released by activated peripheral immune cells during inflammation and infection interact with central neural systems via humoral and vagal afferent pathways. Previous work could characterize critical brain mechanisms that are engaged during systemic inflammation in animals and humans. Via these pathways, cytokines also modulate learning and memory but virtually nothing is known about effects of inflammatory processes on extinction learning. Therefore, A12 will address effects of an experimentally-induced inflammatory response on different aspects of extinction learning in humans and elucidate the afferent pathways and central networks involved. Since inflammation affects neural and subjective measures of pain, A12 will bridge the clinical fields of inflammation and pain. Behavioral conditioning of immune functions constitutes a complementary approach to delineate CNS-immune interactions. Indeed, behaviorally conditioned immune responses can influence severity, progress and mortality rates in experimental animals with chronic inflammatory diseases or cancer. Relevant for the SFB is the finding that extinction of a conditioned suppression of T cell functions can be abrogated or even prevented by reconsolidation or memory up-dating processes. These data introduce the fascinating perspective of interfering with the extinction of learned immunosuppressive effects to employ learning paradigms in clinical conditions as supportive therapy. This idea is the starting point of A18 which aims to solve the following dilemma: under certain clinical conditions patients receive continuous immunosuppressive treatment. Hence, in this specific clinical context, extinction of behaviorally conditioned immunosuppression is undesirable. This is opposite from treatments of anxiety disorders and thus complements other clinically oriented projects of the SFB 1280.
Understanding inter-individual variability and developmental changes in extinction efficacy is crucial. Impaired extinction contributes to pathology and/or to clinically-relevant markers in healthy individuals.
Clinical implications
An understanding of the ontogenetic development and inter-individual differences in extinction efficacy in healthy participants is relevant to comprehend abnormal extinction in patients. The SFB 1280 will therefore implement a neurodevelopmental approach as well as use inter-individual differences as a window into the behavioral and neural mechanisms of extinction learning to gain mechanistic knowledge for clinical applications. Consequently, the research council proposes to focus on individual differences in functional and structural extinction network activity and connectivity both within specific projects (A03, A08) and across all human imaging projects (Focus Group “Neuroimaging” F02). In parallel, they will assess the contribution of sex and age as group-factors in extinction learning within several projects (A09, A10, A11, A16). The clinically-oriented projects of the SFB 1280 target anxiety, pain and immune-related conditions. These conditions, albeit diverse in their clinical presentations, share crucial factors that connect them with parallel approaches carried out in healthy individuals addressing inter-individual variability. A09, A10 and A11 will test effects of acute stress or stress hormones on reinstatement of conditioned fear. This will extend existing data showing reinstatement in visceral pain- and somatic pain-related conditioning paradigms. In patients suffering from anxiety, one project (A13) will ascertain whether the application of a psychosocial stressor prior to exposure therapy improves therapeutic outcome. This is based on treatment studies in anxiety that revealed that cortisol enhances the efficacy of extinction-based therapies presumably by impairing the original fear memory and boosting extinction consolidation. Together, these findings will provide a sophisticated understanding of the mechanisms and implications underlying the role of stress in normal and abnormal extinction learning. Finally, anxiety, pain and auto-immune/inflammatory disorders are more prevalent in women than in men. This builds on existing findings regarding sex differences and the role of sex hormones in the broader fear conditioning literature and data from the groups within the SFB 1280 using electric shock or pain as US. Understanding sex differences in fear conditioning and extinction will improve the understanding of the mechanisms underlying variability in extinction in normals and clinical populations where anxiety plays a role. It will also enable individualized extinction-based interventions such as exposure therapy in the treatment of anxiety and chronic pain.
References
Albring A, Wendt L, Benson S, Nissen S, Yavuz Z, Engler H, Witzke O, Schedlowski M (2014) Preserving learned immunosuppressive placebo response: perspectives for clinical application. Clin Pharmacol Ther. 96: 247–255.
An B, Hong I, Choi S (2012) Long-term neural correlates of reversible fear learning in the lateral amygdala. J Neurosci. 32: 16845–16856.
André MA, Manahan-Vaughan D (2015) Involvement of dopamine D1/D5 and D2 receptors in context-dependent extinction learning and memory reinstatement. Front Behav.Neurosci 9: 372
André MA, Manahan-Vaughan D (2016) Involvement of dopamine D1/D5 and D2 receptors in context-dependent extinction learning and memory reinstatement. Frontiers Behav Neurosci. 9:125. doi: 10.3389/fnbeh.2015.00125
Arbuthnott GW (2014) Thalamostriatal synapses-another substrate for dopamine action? Prog Brain Res. 211: 1–11.
Bzdok, Danilo; Laird, Angela R.; Zilles, Karl; Fox, Peter T.; Eickhoff, Simon B. (2013): An investigation of the structural, connectional, and functional subspecialization in the human amygdala. In: Human brain mapping 34 (12), S. 3247-3266. DOI: 10.1002/hbm.22138.
Benson S, Kattoor J, Kullmann JS, Hofmann S, Engler H, Forsting M, Gizewski ER, Elsenbruch S (2014) Towards understanding sex differences in visceral pain: enhanced reactivation of classically-conditioned fear in healthy women. Neurobiol Learn Mem. 109: 113–121.
Benson S, Kattoor J, Wegner A, Hammes F, Reidick D, Grigoleit J, Engler H, Oberbeck R, Schedlowski M, Elsenbruch S (2012) Acute experimental endotoxemia induces visceral hypersensitivity and altered pain evaluation in healthy humans. Pain. 153: 794–799.
Benson S, Rebernik L, Wegner A, Kleine-Borgmann J, Engler H, Schlamann M, Forsting M, Schedlowski M, Elsenbruch S (2015) Neural circuitry mediating inflammation-induced central pain amplification in human experimental endotoxemia. Brain Behav Immun. 48: 222–231.
Bocchio, M.; McHugh, S. B.; Bannerman, D. M.; Sharp, T.; Capogna, M.: Serotonin, Amygdala and Fear: Assembling the Puzzle. In: Frontiers in neuronal circuits 10 (24). DOI: 10.3389/fncir.2016.00024.
Bostan AC, Dum RP, Strick PL (2013) Cerebellar networks with the cerebral cortex and basal ganglia. Trends Cogn Sci (Regul Ed). 17: 241–254.
Bouton ME (2004) Context and behavioral processes in extinction. Learn Mem. 11: 485–494.
Bouton ME, Westbrook RF, Corcoran KA, Maren S (2006) Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol Psychiatry. 60: 352–360.
Brischoux F, Chakraborty S, Brierley DI, Ungless MA (2009) Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci U S A. 106: 4894–4899.
Chang D, Lissek S, Ernst TM, Thürling M, Üngör M, Tegenthoff M, Ladd ME, Timmann D (2015) Cerebellar Contribution to Context Processing in Extinction Learning and Recall. Cerebellum. doi: 10.1007/s12311-015-0670-z.
Cheng S (2013) The CRISP theory of hippocampal function in episodic memory. Front Neural Circuits. 7: 88.
Cohen JY, Haesler S, Vong L, Lowell BB, Uchida N (2012) Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature. 482: 85–88.
de Quervain, Dominique J-F, Bentz D, Michael T, Bolt OC, Wiederhold BK, Margraf J, Wilhelm FH (2011) Glucocorticoids enhance extinction-based psychotherapy. Proc Natl Acad Sci U S A. 108: 6621–6625.
de Quervain, Dominique J-F, Margraf J (2008) Glucocorticoids for the treatment of post-traumatic stress disorder and phobias: a novel therapeutic approach. Eur J Pharmacol. 583: 365–371.
Doig, Natalie M.; Moss, Jonathan; Bolam, J. Paul (2010): Cortical and thalamic innervation of direct and indirect pathway medium-sized spiny neurons in mouse striatum. In: The Journal of neuroscience : the official journal of the Society for Neuroscience 30 (44), S. 14610–14618. DOI: 10.1523/JNEUROSCI.1623-10.2010.
Elsenbruch S, Wolf OT (2015) Could Stress Contribute to Pain-Related Fear in Chronic Pain? Front Behav Neurosci. 9: 340.
Enck P, Bingel U, Schedlowski M, Rief W (2013) The placebo response in medicine: minimize, maximize or personalize? Nat Rev Drug Discov. 12: 191–204.
Engler H, Doenlen R, Engler A, Riether C, Prager G, Niemi M, Pacheco-López G, Krügel U, Schedlowski M (2011) Acute amygdaloid response to systemic inflammation. Brain Behav Immun. 25: 1384–1392.
Finger EC, Mitchell, Derek G V, Jones M, Blair, R J R (2008) Dissociable roles of medial orbitofrontal cortex in human operant extinction learning. Neuroimage. 43: 748–755.
Forkmann K, Wiech K, Sommer T, Bingel U (2015) Reinstatement of pain-related brain activation during the recognition of neutral images previously paired with nociceptive stimuli. Pain. 156: 1501–1510.
Gerwig M, Hajjar K, Frings M, Dimitrova A, Thilmann AF, Kolb FP, Forsting M, Timmann D (2006) Extinction of conditioned eyeblink responses in patients with cerebellar disorders. Neurosci Lett. 406: 87–91.
Grahame NJ, Hallam SC, Geier L, Miller RR (1990) Context as an occasion setter following either CS acquisition and extinction or CS acquisition alone. Learning and Motivation. 21: 237–265.
Guindon J, Hohmann AG (2009) The Endocannabinoid System and Pain. CNS Neurol Disord Drug Targets. 8: 403–421.
Haaker J, Gaburro S, Sah A, Gartmann N, Lonsdorf TB, Meier K, Singewald N, Pape HC, Morellini F, Kalisch R (2013) Single dose of L-dopa makes extinction memories context-independent and prevents the return of fear. PNAS 110: E2428-E2436.
Hadamitzky M, Engler H, Schedlowski M (2013) Learned immunosuppression: extinction, renewal, and the challenge of reconsolidation. J Neuroimmun Pharmacol. 8: 180–188.
Hagena H, Manahan-Vaughan D (2016) The serotonergic 5-HT4 receptor: a unique modulator of hippocampal synaptic information processing and cognition, Neurobiol. Learn. Mem. Jun 15. pii: S1074-7427(16)30089-2; doi: 10.1016/j.nlm.2016.06.014. [Epub ahead of print].
Hall G, Honey RC (1990) Context-specific conditioning in the conditioned-emotional-response procedure. Journal of Experimental Psychology: Animal Behavior Processes. 16: 271–278.
Hamacher-Dang TC, Engler H, Schedlowski M, Wolf OT (2013) Stress enhances the consolidation of extinction memory in a predictive learning task. Front Behav Neurosci. 7: 108.
Hamacher-Dang TC, Merz CJ, Wolf OT (2015) Stress following extinction learning leads to a context-dependent return of fear. Psychophysiology. 52: 489–498.
Harris, Kenneth D.; Shepherd, Gordon M. G. (2015): The neocortical circuit: themes and variations. In: Nature neuroscience 18 (2), S. 170-181. DOI: 10.1038/nn.3917.
Hennigan K, D’Ardenne K, McClure SM (2015) Distinct midbrain and habenula pathways are involved in processing aversive events in humans. J Neurosci. 35: 198–208.
Herry C, Ciocchi S, Senn V, Demmou L, Müller C, Lüthi A (2008) Switching on and off fear by distinct neuronal circuits. Nature. 454: 600–606.
Hirsh R (1974) The hippocampus and contextual retrieval of information from memory: A theory. Behavioral Biology. 12: 421–444.
Hobin JA, Goosens KA, Maren S (2003) Context-dependent neuronal activity in the lateral amygdala represents fear memories after extinction. J Neurosci. 23: 8410–8416.
Howlett AC, Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Porrino LJ (2004) Cannabinoid physiology and pharmacology: 30 years of progress. Neuropharmacology. 47 Suppl 1: 345–358.
Icenhour A, Langhorst J, Benson S, Schlamann M, Hampel S, Engler H, Forsting M, Elsenbruch S (2015a) Neural circuitry of abdominal pain-related fear learning and reinstatement in irritable bowel syndrome. Neurogastroenterol Motil. 27: 114–127.
Icenhour A, Kattoor J, Benson S, Boekstegers A, Schlamann M, Merz CJ, Forsting M, Elsenbruch S (2015b) Neural circuitry underlying effects of context on human pain-related fear extinction in a renewal paradigm. Hum Brain Mapp. 36: 3179–3193.
Joëls M, Pu Z, Wiegert O, Oitzl MS, Krugers HJ (2006) Learning under stress: how does it work? Trends Cogn Sci (Regul Ed). 10: 152–158.
Kattoor J, Thürling M, Gizewski ER, Forsting M, Timmann D, Elsenbruch S (2014) Cerebellar contributions to different phases of visceral aversive extinction learning. Cerebellum. 13: 1–8.
Kemp A, Manahan-Vaughan D. (2005) The 5-hydroxytryptamine4 receptor exhibits frequency-dependent properties in synaptic plasticity and behavioural metaplasticity in the hippocampal CA1 region in vivo.Cereb Cortex. 15:1037-43.
Kim JH, Richardson R (2008) The effect of temporary amygdala inactivation on extinction and reextinction of fear in the developing rat: unlearning as a potential mechanism for extinction early in development. J Neurosci. 28: 1282–1290.
Kinner VL, Merz CJ, Lissek S, Wolf OT (2016) Cortisol disrupts the neural correlates of extinction recall. Neuroimage 133: 233-243.
Labrenz F, Icenhour A, Thürling M, Schlamann M, Forsting M, Timmann D, Elsenbruch S (2015) Sex differences in cerebellar mechanisms involved in pain-related safety learning. Neurobiol Learn Mem. 123: 92–99.
Labrenz F, Icenhour A, Schlamann M, Forsting M, Bingel U, Elsenbruch S (2016) From Pavlov to pain: How predictability affects the anticipation and processing of visceral pain in a fear conditioning paradigm. Neuroimage. 130: 104-14.
Lacey, Carolyn J.; Bolam, J. Paul; Magill, Peter J. (2007): Novel and distinct operational principles of intralaminar thalamic neurons and their striatal projections. In: The Journal of neuroscience : the official journal of the Society for Neuroscience 27 (16), S. 4374–4384. DOI: 10.1523/JNEUROSCI.5519-06.2007.
Lang S, Kroll A, Lipinski SJ, Wessa M, Ridder S, Christmann C, Schad LR, Flor H (2009) Context conditioning and extinction in humans: differential contribution of the hippocampus, amygdala and prefrontal cortex. Eur J Neurosci. 29: 823–832.
Lengersdorf D, Stüttgen MC, Üngör M, Güntürkün O (2014) Transient inactivation of the pigeon hippocampus or the nidopallium caudolaterale during extinction learning impairs extinction retrieval in an appetitive conditioning paradigm. Behav Brain Res. 265: 93–100.
Lissek S, Glaubitz B, Güntürkün O, Tegenthoff M (2015) Noradrenergic stimulation modulates activation of extinction-related brain regions and enhances contextual extinction learning without affecting renewal. Front Behav Neurosci. 9: 34.
Lissek S, Glaubitz B, Üngör M, Tegenthoff M (2013) Hippocampal activation during extinction learning predicts occurrence of the renewal effect in extinction recall. Neuroimage. 81: 131–143.
Lissek S, Glaubitz B, Schmidt-Wilcke T, Tegenthoff M (2016) Hippocampal Context Processing during Acquisition of a Predictive Learning Task Is Associated with Renewal in Extinction Recall. J Cogn Neurosci 28:5 747–762.
Lissek S, Glaubitz B, Wolf OT, Tegenthoff M (2015b) The DA antagonist tiapride impairs context-related extinction learning in a novel context without affecting renewal. Front Behav Neurosci 9: 238.
Lonsdorf TB, Kalisch R (2011) A review on experimental and clinical genetic associations studies on fear conditioning, extinction and cognitive-behavioral treatment. Transl Psychiatry 1: e41.
Lucke S, Lachnit H, Koenig S, Üngör M (2013) The informational value of contexts affects context-dependent learning. Learn Behav. 41: 285–297.
Lucke S, Lachnit H, Stüttgen MC, Üngör M (2014) The impact of context relevance during extinction learning. Learn Behav. 42: 256–269.
Magal A, Mintz M (2014) Inhibition of the amygdala central nucleus by stimulation of cerebellar output in rats: a putative mechanism for extinction of the conditioned fear response. Eur J Neurosci. 40: 3548–3555.
Mallan KM, Lipp OV, Cochrane B (2013) Slithering snakes, angry men and out-group members: what and whom are we evolved to fear? Cogn Emot. 27: 1168–1180.
Maren S, Chang C (2006) Recent fear is resistant to extinction. Proc Natl Acad Sci U S A. 103: 18020–18025.
Maren, Stephen; Holmes, Andrew (2016): Stress and Fear Extinction. In: Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 41 (1), S. 58-79. DOI: 10.1038/npp.2015.180.
Maruki K, Izaki Y, Akema T, Nomura M (2003) Effects of acetylcholine antagonist injection into the prefrontal cortex on the progress of lever-press extinction in rats. Neurosci Lett. 351: 95–98.
Matsumoto M, Hikosaka O (2007) Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 447: 1111–1115.
McGaugh JL, Roozendaal B (2002) Role of adrenal stress hormones in forming lasting memories in the brain. Curr Opin Neurobiol 12: 205-210.
McHaffie JG, Stanford TR, Stein BE, Coizet V, Redgrave P (2005) Subcortical loops through the basal ganglia. Trends Neurosci. 28: 401–407.
Medina JF, Nores WL, Mauk MD (2002) Inhibition of climbing fibres is a signal for the extinction of conditioned eyelid responses. Nature. 416: 330–333.
Meir Drexler S, Merz CJ, Hamacher-Dang TC, Tegenthoff M, Wolf OT (2015) Effects of Cortisol on Reconsolidation of Reactivated Fear Memories. Neuropsychopharmacology. doi: 10.1038/npp.2015.160
Merz CJ, Hamacher-Dang TC, Wolf OT (2014) Exposure to stress attenuates fear retrieval in healthy men. Psychoneuroendocrinology. 41: 89–96.
Merz CJ, Hermann A, Stark R, Wolf OT (2014) Cortisol modifies extinction learning of recently acquired fear in men. Soc Cogn Affect Neurosci. 9: 1426–1434.
Merz CJ, Tabbert K, Schweckendiek J, Klucken T, Vaitl D, Stark R, Wolf OT (2012) Oral contraceptive usage alters the effects of cortisol on implicit fear learning. Horm Behav. 62: 531–538.
Merz CJ, Wolf OT, Schweckendiek J, Klucken T, Vaitl D, Stark R (2013) Stress differentially affects fear conditioning in men and women. Psychoneuroendocrinology. 38: 2529–2541.
Minai AA, Barrows GL, Levy WB (1994) Disambiguation of pattern sequences with recurrent networks. In International Neural Network Society (Boston) (Ed.) World Congress on Neural Networks. Boston, Hillsdale NJ : L. Erlbaum: 176–181.
Morris RW, Bouton ME (2007) The effect of yohimbine on the extinction of conditioned fear: a role for context. Behav Neurosci 121:, 501-514.
Mühlberger A, Wiedemann G, Herrmann MJ, Pauli P (2006) Phylo- and ontogenetic fears and the expectation of danger: differences between spider- and flight-phobic subjects in cognitive and physiological responses to disorder-specific stimuli. J Abnorm Psychol. 115: 580–589.
Myers CE, Gluck MA (1994) Context, conditioning, and hippocampal rerepresentation in animal learning. Behav Neurosci. 108: 835–847.
Nadel L, Willner J (1980) Context and conditioning: A place for space. Psychobiology. 8: 218–228.
Nakajima S, Tanaka S, Urushihara K, Imada H (2000) Renewal of Extinguished Lever-Press Responses upon Return to the Training Context. Learning and Motivation. 31: 416–431.
Namburi P, Beyeler A, Yorozu S, Calhoon GG, Halbert SA, Wichmann R, Holden SS, Mertens KL, Anahtar M, Felix-Ortiz AC, Wickersham IR, Gray JM, Tye KM (2015) A circuit mechanism for differentiating positive and negative associations. Nature. 520: 675–678.
Nemeroff CB (2002) Recent advances in the neurobiology of depression. Psychopharmacol Bull 36 Suppl 2: 6-23.
Ohman, A.; Mineka, S. (2001): Fears, phobias, and preparedness: toward an evolved module of fear and fear learning. In: Psychological review 108 (3), S. 483-522.
O’Keefe J, Dostrovsky J (1971) The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Research. 34: 171–175.
Pabba, Mohan (2013): Evolutionary development of the amygdaloid complex. In: Frontiers in neuroanatomy 7, S. 27. DOI: 10.3389/fnana.2013.00027.
Pape H, Pare D (2010) Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev. 90: 419–463.
Parent M, Parent A (2005) Single-axon tracing and three-dimensional reconstruction of centre median-parafascicular thalamic neurons in primates. J Comp Neurol. 481: 127–144.
Paton JJ, Belova MA, Morrison SE, Salzman CD (2006) The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature. 439: 865–870.
Petrovich GD, Canteras NS, Swanson LW (2001) Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Research Reviews. 38: 247–289.
Prager G, Hadamitzky M, Engler A, Doenlen R, Wirth T, Pacheco-López G, Krügel U, Schedlowski M, Engler H (2013) Amygdaloid signature of peripheral immune activation by bacterial lipopolysaccharide or staphylococcal enterotoxin B. J Neuroimmune Pharmacol. 8: 42–50.
Quirk GJ, Russo GK, Barron JL, Lebron K (2000) The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci. 20: 6225–6231.
Redish AD, Jensen S, Johnson A, Kurth-Nelson Z (2007) Reconciling reinforcement learning models with behavioral extinction and renewal: implications for addiction, relapse, and problem gambling. Psychological Review. 114: 784–805.
Redondo RL, Kim J, Arons AL, Ramirez S, Liu X, Tonegawa S (2014) Bidirectional switch of the valence associated with a hippocampal contextual memory engram. Nature. 513: 426–430.
Rescorla RA (2004) Spontaneous recovery varies inversely with the training-extinction interval. Animal Learning & Behavior. 32: 401–408.
Rescorla RA (2008) Within-subject renewal in sign tracking. Q J Exp Psychol (Hove). 61: 1793–1802.
Rescorla RA, Wagner AR (1972) A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In Black, AH, Prokasy, WF (Ed.) Appleton-Century-Crofts: 64–99.
Rhodes, Sarah E V, Killcross S (2004) Lesions of rat infralimbic cortex enhance recovery and reinstatement of an appetitive Pavlovian response. Learn Mem. 11: 611–616.
Roozendaal B, McEwen BS, Chattarji S (2009) Stress, memory and the amygdala. Nat Rev Neurosci. 10: 423–433.
Schedlowski M, Enck P, Rief W, Bingel U (2015) Neuro-Bio-Behavioral Mechanisms of Placebo and Nocebo Responses: Implications for Clinical Trials and Clinical Practice. Pharmacol Rev. 67: 697–730.
Schedlowski M, Pacheco-López G (2010) The learned immune response: Pavlov and beyond. Brain Behav Immun. 24: 176–185.
Schiller D, Levy I, Niv Y, Ledoux JE, Phelps EA (2008) From fear to safety and back: reversal of fear in the human brain. J Neurosci. 28: 11517–11525.
Schmidt K, Forkmann K, Sinke C, Gratz M, Bitz A, Bingel U. (2016) The differential effect of trigeminal vs. peripheral pain stimulation on visual processing and memory encoding is influenced by pain-related fear. Neuroimage. Jul 1;134:386-95.
Schultz W (2016) Dopamine reward prediction-error signalling: a two-component response. Nat Rev Neurosci 17: 183-195.
Seehagen S, Schneider S, Rudolph J, Ernst S, Zmyj N (2015) Stress impairs cognitive flexibility in infants. Proc Natl Acad Sci USA. 112(41):12882–128826.
Shabel SJ, Janak PH (2009) Substantial similarity in amygdala neuronal activity during conditioned appetitive and aversive emotional arousal. Proc Natl Acad Sci U S A. 106: 15031–15036.
Song EY, Boatman JA, Jung MW, Kim JJ (2010) Auditory Cortex is Important in the Extinction of Two Different Tone-Based Conditioned Fear Memories in Rats. Front Behav Neurosci. 4: 24.
Sotres-Bayon F, Cain CK, Ledoux JE (2006) Brain mechanisms of fear extinction: historical perspectives on the contribution of prefrontal cortex. Biol Psychiatry. 60: 329–336.
Spoida K, Masseck OA, Deneris ES, Herlitze S (2014) Gq/5-HT2c receptor signals activate a local GABAergic inhibitory feedback circuit to modulate serotonergic firing and anxiety in mice. PNAS 111: 6479-6484.
Starosta S, Bartetzko I, Lucke S, Uengoer M, Güntürkün O, Stüttgen MC (2016) Context-specificity of both acquisition and extinction of a Pavlovian conditioned response, Learning and Memory, October 17 [EPub].
Stockhorst U, Antov MI (2015) Modulation of Fear Extinction by Stress, Stress Hormones and Estradiol: A Review. Front Behav Neurosci 9: 359.
Sutton RS, Barto AG (1987) A temporal-difference model of classical conditioning. Proc Annu Conf Cogn Sci Soc: 355–378.
Swanson LW, Petrovich GD (1998) What is the amygdala? Trends Neurosci. 21: 323–331.
Thürling M, Kahl F, Maderwald S, Stefanescu RM, Schlamann M, Boele H, De Zeeuw, Chris I, Diedrichsen J, Ladd ME, Koekkoek, Sebastiaan K E, Timmann D (2015) Cerebellar cortex and cerebellar nuclei are concomitantly activated during eyeblink conditioning: a 7T fMRI study in humans. J Neurosci. 35: 1228–1239.
Tracey KJ (2010) Understanding immunity requires more than immunology. Nat Immunol. 11: 561–564.
Utz A, Thurling M, Ernst TM, Hermann A, Stark R, Wolf OT, Timmann D, Merz CJ (2015) Cerebellar vermis contributes to the extinction of conditioned fear. Neurosci Lett. 604: 173–177.
Viviani, Daniele; Charlet, Alexandre; van den Burg, Erwin; Robinet, Camille; Hurni, Nicolas; Abatis, Marios et al. (2011): Oxytocin selectively gates fear responses through distinct outputs from the central amygdala. In: Science (New York, N.Y.) 333 (6038), S. 104-107. DOI: 10.1126/science.1201043.
Wegner A, Elsenbruch S, Maluck J, Grigoleit J, Engler H, Jager M, Spreitzer I, Schedlowski M, Benson S (2014) Inflammation-induced hyperalgesia: effects of timing, dosage, and negative affect on somatic pain sensitivity in human experimental endotoxemia. Brain Behav Immun. 41: 46–54.
Williams AE, Rhudy JL (2007) The influence of conditioned fear on human pain thresholds: does preparedness play a role? J Pain. 8: 598–606.
Wolf OT (2009) Stress and memory in humans: twelve years of progress? Brain Research. 1293: 142–154.
Woods AM, Bouton ME (2008) Immediate extinction causes a less durable loss of performance than delayed extinction following either fear or appetitive conditioning. Learn Mem. 15: 909–920.